Alkaline batteries stand as a testament to modern technology, providing reliable energy for countless devices. I find it fascinating that the global annual production volume of alkaline batteries exceeds 15 billion units, highlighting their widespread use. These batteries are produced by skilled manufacturers through a meticulous manufacturing process, which involves the careful selection of materials and precise chemical reactions. This attention to detail ensures that they deliver consistent performance in various applications, from household gadgets to essential electronics.
Key Takeaways
- Alkaline batteries are made from key components like zinc, manganese dioxide, and potassium hydroxide, each playing a crucial role in energy production.
- The manufacturing process includes careful preparation of raw materials, mixing, and assembly, ensuring high-quality and reliable batteries.
- Understanding the chemical reactions in alkaline batteries helps appreciate how they generate electricity, with zinc oxidizing at the anode and manganese dioxide reducing at the cathode.
- Choosing a reputable manufacturer, like Ningbo Johnson New Eletek, ensures quality products and support, which is vital for industries relying on battery performance.
- Proper disposal and recycling of alkaline batteries are essential for environmental protection, so always follow local regulations.
Components of Alkaline Batteries
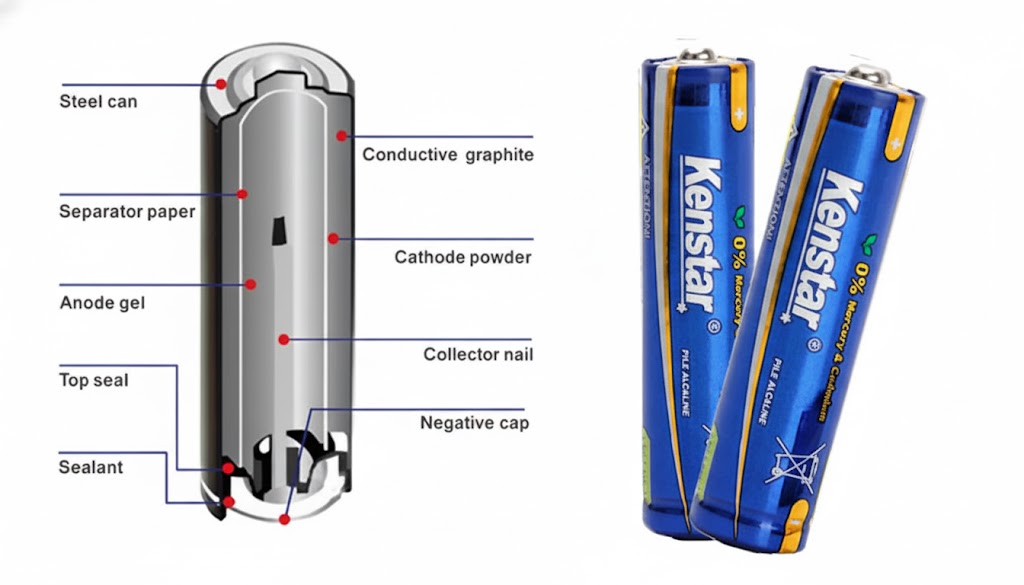
Alkaline batteries consist of several key components, each playing a vital role in their functionality. Understanding these components helps me appreciate how they work together to produce reliable energy. Here’s a breakdown of the primary materials used in the construction of alkaline batteries:
| Material | Role in Battery Construction |
|---|---|
| Zinc | Acts as the anode, providing the necessary electrons |
| Manganese Dioxide (MnO2) | Serves as the cathode material |
| Potassium Hydroxide (KOH) | Functions as the alkaline electrolyte |
| Steel | Forms the battery’s body and serves as the cathode |
| Conductive Graphite | Enhances conductivity within the battery |
| Separator Paper | Prevents short-circuiting between anode and cathode |
| Sealing Plug | Ensures the integrity of the battery’s contents |
Zinc is crucial as it forms the anode in alkaline batteries. It oxidizes during discharge, producing zinc oxide and releasing electrons. The performance of the battery largely depends on the properties of the zinc used. For instance, the particle size and shape of zinc powder can significantly affect the battery’s capacity and longevity.
Manganese dioxide serves as the cathode material. This configuration allows for a higher capacity compared to standard zinc-carbon cells. It is essential for the electrochemical reactions that generate electrical energy. The combination of manganese dioxide with graphite improves conductivity, enhancing overall battery performance.
Potassium hydroxide acts as the electrolyte, enabling the flow of ions between the anode and cathode. This ion transport is vital for sustaining the chemical reactions that produce electricity. Additionally, potassium hydroxide helps maintain charge balance within the battery, ensuring stable operation.
The steel casing not only provides structural integrity but also serves as a cathode. The separator paper is another critical component, preventing short-circuiting between the anode and cathode, which could lead to battery failure. Finally, the sealing plug ensures that the battery’s contents remain intact, preventing leakage and maintaining performance.
The Manufacturing Process

The manufacturing process of alkaline batteries is intricate and involves several critical steps. Each phase contributes to the overall efficiency and reliability of the final product. I find it fascinating how these steps come together to create a power source that we often take for granted.
Raw Material Preparation
The journey begins with the careful preparation of raw materials. I have learned that sourcing these materials is essential for producing high-quality batteries. Here’s how it unfolds:
- Zinc Extraction: Zinc is extracted from ore, often alongside other elements. This process generates a high-grade zinc concentrate, which is crucial for the anode.
- Manganese Dioxide and Carbon: For the cathode, manufacturers granulate manganese dioxide and mix it with carbon. This mixture is then pressed into preforms.
- Electrolyte Solution: Potassium hydroxide is measured and prepared to facilitate ion flow within the battery.
- Separator Production: The separator, made from paper or synthetic fiber, is manufactured to prevent short circuits between the anode and cathode.
This meticulous preparation ensures that the materials meet the necessary specifications for optimal battery performance.
Mixing and Forming
Once the raw materials are ready, the next step involves mixing and forming the active materials. I find this phase particularly interesting because it sets the stage for the battery’s chemical reactions. The process includes:
- Mixing Equipment: Various machines, such as lab mixers and planetary ball mills, are employed to create a uniform mixture of zinc powder and potassium hydroxide for the anode.
- Cathode Formation: The manganese dioxide and carbon mixture undergoes granulation and is then pressed into the desired shape.
- Gel Creation: The anode material is transformed into a gel-like consistency, which enhances its performance during discharge.
This phase is crucial as it directly impacts the battery’s capacity and longevity.
Assembly Line Operations
The final stage of the manufacturing process occurs on the assembly line. This is where automation plays a significant role in maximizing productivity. I have observed that the assembly line operations consist of several key steps:
- Steel Can Preparation: The steel can, which serves as the negative terminal, is prepared for assembly.
- Gel Insertion: The gel created from zinc powder and potassium hydroxide is inserted into the can.
- Separator Placement: A separator paper is placed to prevent any short circuits.
- Cathode Insertion: The manganese dioxide cathode material is inserted around a carbon rod current collector.
Automation technologies, such as robotic arms and automated assembly systems, streamline these operations. This not only enhances efficiency but also reduces labor costs. I appreciate how AI-driven analytics optimize production lines, minimizing waste and operational costs. Predictive maintenance powered by AI anticipates equipment failures, ensuring smooth operations.
Finally, end-of-line (EOL) testing is performed to verify that each battery meets the required specifications. This testing checks critical parameters like voltage and resistance, ensuring that only high-quality products reach consumers.
Chemical Reactions in Alkaline Batteries
The chemical reactions in alkaline batteries fascinate me. They are the heart of how these batteries generate electricity. Understanding these reactions helps me appreciate the science behind the power sources we often take for granted.
In alkaline batteries, two primary reactions occur: oxidation at the anode and reduction at the cathode. The anode reaction involves zinc, which oxidizes to produce zinc oxide while releasing electrons. This process is crucial because it generates the flow of electrons that powers our devices. The cathode reaction involves manganese dioxide, which undergoes reduction in the presence of water and electrons. This reaction forms manganese oxide and hydroxide ions.
Here’s a table summarizing these reactions:
| Reaction Type | Reaction |
|---|---|
| Cathode (reduction) | [\ce{2MnO2(s) + H2O(l) + 2e^{−} -> Mn2O3(s) + 2OH^{−}(aq)}] |
| Anode (oxidation) | [\ce{Zn(s) + 2OH^{−}(aq) -> ZnO(s) + H2O(l) + 2e^{−}}] |
| Overall Reaction | [\ce{Zn(s) + 2MnO2(s) -> ZnO(s) + Mn2O3(s)}] |
The overall reaction combines both processes, illustrating how zinc and manganese dioxide work together to produce energy.
I find it interesting that alkaline batteries use potassium hydroxide (KOH) as their electrolyte. This differs from non-alkaline batteries, which often utilize zinc chloride (ZnCl2). This difference in chemical composition leads to distinct reactions, affecting the performance and longevity of the batteries. The use of KOH allows for a more efficient ion flow, contributing to the higher energy density that alkaline batteries are known for.
Types of Alkaline Batteries
Alkaline batteries come in two primary types: standard alkaline batteries and rechargeable alkaline batteries. Each type serves different purposes and applications, making them essential in our daily lives.
Standard Alkaline Batteries
Standard alkaline batteries are the most common type found in households. They provide a voltage of 1.5V, making them suitable for various low-power devices. I often use them in remote controls, clocks, and toys. Their versatility is impressive, as they power many everyday gadgets. Here’s a quick overview of their typical applications:
- Remote controls
- Clocks
- Wireless peripherals
- Toys
- Flashlights
- Medical devices
The table below summarizes the sizes and applications of standard alkaline batteries:
| Size | Application |
|---|---|
| AA | Household items, toys, flashlights |
| AAA | Digital cameras, MP3 players |
| C | High drain devices |
| D | Low drain devices |
| Other | Various household applications |
Rechargeable Alkaline Batteries
Rechargeable alkaline batteries offer a more sustainable option. While they typically provide a lower voltage of 1.2V, this difference does not hinder their performance in low-drain devices. I find them particularly useful for applications where I frequently replace batteries. These batteries can be recharged hundreds of times, making them both cost-effective and environmentally friendly.
Rechargeable alkaline batteries are often made from nickel–metal hydride (NiMH) and are designed to be chemically sealed. This design helps prevent leakage, a common issue with standard batteries. Their efficiency and longevity make them an excellent choice for high-drain devices like digital cameras and gaming controllers.
Manufacturer Spotlight: Ningbo Johnson New Eletek Co., Ltd.
Ningbo Johnson New Eletek Co., Ltd. has made a significant mark in the alkaline battery manufacturing sector since its establishment in 2004. I admire how this manufacturer focuses on producing high-quality, reliable batteries while committing to sustainable development and eco-friendly practices. Their emphasis on mutual benefit and long-term partnerships has helped them build trust with clients globally.
Here’s a quick overview of the company’s key aspects:
| Aspect | Details |
|---|---|
| Founded | 2004 |
| Fixed Assets | $5 million |
| Production Workshop Area | 10,000 square meters |
| Number of Employees | 200 |
| Production Lines | 8 fully automatic lines |
I appreciate that Johnson New Eletek operates with a smaller scale compared to larger manufacturers, yet they excel in product quality. Their automated production lines enhance efficiency, allowing them to maintain high standards. The company prioritizes eco-friendly innovations in battery production, which resonates with my values.
In terms of quality assurance, Johnson New Eletek adheres to several certifications and standards. They have passed the ISO9001 quality certification, ensuring high reliability in their products. Additionally, they continuously improve their production technology in strict accordance with ISO 9001:2000 standards.
To illustrate their competitive edge, I found a comparison of Johnson New Eletek with other leading manufacturers:
| Supplier Name | Review Scores | On-Time Delivery | Online Revenue | Reorder Rate |
|---|---|---|---|---|
| Ningbo Johnson New Eletek Co., Ltd. | 4.9/5.0 | 96.8% | $255,000+ | 19% |
| Zhongyin (Ningbo) Battery Co., Ltd. | 5.0/5.0 | 98.2% | $990,000+ | 16% |
| Ningbo Mustang International Trade Co. | 5.0/5.0 | 97.5% | $960,000+ | 22% |
This data shows that while Johnson New Eletek may not lead in revenue, their commitment to quality and customer satisfaction is evident in their high review scores. Choosing a manufacturer like Johnson New Eletek means opting for quality products at competitive prices, backed by a professional sales team ready to assist clients worldwide.
The manufacturing of alkaline batteries is a complex process that combines various materials and chemical reactions. This results in efficient energy sources for everyday use. I believe that understanding this process enhances our appreciation for the batteries we often take for granted.
When selecting a manufacturer for bulk purchases, consider factors such as quality control, in-process monitoring, and production equipment. A reliable supplier ensures quality products and support services.
Safety is a paramount concern when purchasing batteries, especially for critical industries like healthcare or manufacturing.
Choosing a reputable manufacturer like Ningbo Johnson New Eletek Co., Ltd. guarantees quality and competitive pricing. Their commitment to excellence makes them a trusted partner in the battery industry.
| Key Aspect | Description |
|---|---|
| Quality Control | Comprehensive testing including voltage verification, capacity testing, and leakage resistance testing. |
| In-process Monitoring | Monitoring of key parameters like material distribution and assembly dimensions. |
By prioritizing these factors, I can ensure that I make informed decisions when it comes to battery procurement.
FAQ
What is the lifespan of an alkaline battery?
Alkaline batteries typically last between 3 to 10 years, depending on usage and storage conditions. I find that devices with low power consumption extend battery life significantly.
Can I recharge standard alkaline batteries?
No, standard alkaline batteries are not designed for recharging. Attempting to recharge them can lead to leakage or rupture. I recommend using rechargeable alkaline batteries for that purpose.
How should I dispose of alkaline batteries?
I always dispose of alkaline batteries according to local regulations. Many areas have designated recycling programs. I avoid throwing them in regular trash to protect the environment.
Are alkaline batteries safe to use?
Yes, alkaline batteries are generally safe when used correctly. I ensure to follow the manufacturer’s guidelines and avoid mixing old and new batteries to prevent leaks or malfunctions.
What devices commonly use alkaline batteries?
I often find alkaline batteries in various devices, including remote controls, toys, flashlights, and clocks. Their versatility makes them a popular choice for everyday gadgets.
Post time: Oct-09-2025




